news


BioVersys and Partners' Phase 2a Tuberculosis Trial Results Published in New England Journal of Medicine
(19 Feb, 2026) BioVersys AG (SIX: BIOV), a multi-asset, clinical stage biopharmaceutical company focusing on research and development of novel antibacterial products for serious life-threatening infections caused by multidrug-resistant (MDR) bacteria, announced today the publication of promising clinical proof of concept results in the prestigious New England Journal of Medicine from the Phase 2a clinical trial of AlpE in patients with pulmonary TB.


BioVersys Announces BV100 Phase 3 Initiation and Provides a Business Update
(11 December, 2025) BioVersys AG (SIX: BIOV), a multi-asset, clinical stage biopharmaceutical company focusing on research and development of novel antibacterial products for serious life-threatening infections caused by multidrug-resistant (MDR) bacteria, announced today the initiation of its lead asset BV100 Phase 3 clinical program and provides a business update summarizing the Company’s progress in 2025 and the events expected in 2026.

Breaking News: Guangzhou Corebone Obtains Class III Medical Device Certification
(6 June, 2025) Guangzhou Corebone Medical Technology Co., Ltd., a portfolio company of GIBF, announced that its dental bone powder product has officially obtained the Class III Medical Device Registration Certificate issued by the National Medical Products Administration (NMPA).


BioVersys Announces Important BV100 Patent Granted by Chinese Patent Office
(31 March, 2025) BioVersys AG (SIX: BIOV), a multi-asset, clinical stage biopharmaceutical company focusing on research and development of novel antibacterial products for serious life-threatening infections caused by multidrug-resistant (MDR) bacteria, announced today, that the company was granted important patent claims in China for its BV100 technology.


Silexion Therapeutics Announces Completion of Initial Study in Orthotopic Pancreatic Cancer Models Evaluating Systemic Administration of SIL-204
(25 February, 2025) Silexion Completes Data Collection Phase in First-Ever Evaluation of SIL-204 in Clinically Relevant Orthotopic Models; Analysis Underway with Initial Results Expected in Coming Weeks.


BIOVERSYS SUCCESSFULLY ADVANCES BV500 NTM PROGRAM WITH CONTINUED SUPPORT FROM CF AMR SYNDICATE
(11 February, 2025) BioVersys AG (SIX: BIOV) announced today that its BV500 NTM program has reached its second milestone, “identification of up to 5 Optimized Lead compounds”, under the CF AMR Syndicate Collaborative Discovery Programme (CDP) agreement. Supported by LifeArc funding, BioVersys is developing novel small molecules targeting difficult to treat non-tuberculous mycobacteria lung disease (NTM-LD) in people with cystic fibrosis (CF).


Pioneering Biotech Company BioVersys Opens IPO Season at SIX Swiss Exchange
(7 February, 2025) Today, BioVersys (Ticker “BIOV”), a leading Swiss biotech company specializing in novel antibacterial products for serious life-threatening infections, listed its shares at SIX Swiss Exchange. At an opening price of CHF 36.50 per share, the resulting market capitalization of BioVersys was around CHF 216 million.


Silexion Therapeutics Announces Exercise of Warrants for $3.3 Million Gross Proceeds
(29 Janruary, 2025) Silexion Therapeutics Corp. (NASDAQ: SLXN), a clinical-stage biotech developing RNA interference (RNAi) therapies for KRAS-driven cancers, today announced the entry into definitive agreements for the immediate exercise of certain outstanding warrants to purchase up to an aggregate of 2,221,523 of the Company’s ordinary shares originally issued in January 2025 having an exercise price of $1.35 per share.


Silexion Therapeutics Reports Strong Tumor Growth Reduction from Systemic Administration of SIL-204 in Preclinical Pancreatic Cancer Models
(28 Janruary, 2025) New preclinical findings provide validation for Silexion’s new systemic administration approach for SIL-204, demonstrating inhibition of tumor growth in a clinically relevant orthotopic model; Further studies aim to evaluate its impact on metastases.


BIOVERSYS ANNOUNCES INTENTION TO FLOAT ON THE SIX SWISS EXCHANGE
(21 January, 2025) BioVersys AG, a multi-asset, clinical stage biopharmaceutical company focusing on research and development of novel antibacterial products for serious life-threatening infections caused by multidrug-resistant (MDR) bacteria, announced today that it plans to conduct an initial public offering (“IPO”) on the SIX Swiss Exchange in the first quarter of 2025.


Corneat KPro: Successful Implantation of the First Fully Artificial Cornea
(25 October, 2024) Corneat Vision, a project invested by GIBF, recently featured on a France TV show with the story the first French patient who received a new and entirely artificial cornea- the Corneat KPro.


BioVersys Joins Eu-Funded RespiriNTM Programme to Accelerate Development of its Broad-Spectrum Drug Candidates Against NTM Pulmonary Diseases
(22 October, 2024) RespiriNTM Programme allows BioVersys’ NTM project to access up to €2 million of non-dilutive funding.


BioVersys Announces Last Patient Last Visit in BV100 Phase 2 Clinical Trial in Ventilator Associated Bacterial Pneumonia (VABP) and Provides a Business Update
(16 October, 2024) BioVersys's BV100 Phase 2 preliminary results suggest BV100 is generally safe and well tolerated and showed strong initial signs of efficacy in VABP patients.


Silexion Therapeutics Reports Breakthroughs From SIL-204 Preclinical Studies
(1 October, 2024) Latest preclinical studies reveal significant improvements in stability, efficacy, and KRAS targeting range for next-generation siRNA candidate SIL-204.


Silexion Therapeutics Announces Significant New Data from Phase 2 Trial of LODER™ in Non-Resectable Pancreatic Cancer
(24 September, 2024)New analysis from Silexion's Phase 2 trial of LODER shows a 56% objective response rate (ORR) and 67% resectability improvement in non-resectable pancreatic cancer


GIBF Supports BioVersys AG in expanding into the Chinese market as part of its global Antimicrobial Resistance (“AMR”) and tuberculosis strategy
(11 September, 2024) GIBF announced today that it has successfully participated in the Series C+ round of financing of the Swiss-based company, BioVersys AG, with a total investment amount of 6 million US dollars (approx. 42.5 million RMB).


Nectin Therapeutics and Immunome Inc. have reached a collaboration on monoclonal antibodies
Nectin Therapeutics, Ltd., the parent company of Guangzhou Nectin Pharmaceutical Co., Ltd., a project invested by GIBF2 recently announced a global exclusive license agreement with Immunome Inc. for a monoclonal antibody molecule.


Congratulations! Prof. Shlomo Noy, a partner of GIBF, has co-founded the medical device company Innovalve, which has been acquired by the American medical device giant Edwards Lifesciences for $300 million
Recently, the American medical device giant Edwards Lifesciences announced the completion of the acquisition of Innovalve Bio Medical (hereinafter referred to as Innovalve), a spin-off from the Sheba Medical Center, for $300 million in cash. The acquisition plan is expected to be completed by the end of 2024, at which time Innovalve will be integrated into Edwards Lifesciences' Transcatheter Mitral and Tricuspid Valve Therapies (TMVT) 'product group, a news that has attracted widespread attention in the biomedical field.


GIBF invests $10 Million in Nectin Therapeutics to Advance Novel Pipeline of First-in-Class Immunotherapies and Antibody Drug Conjugates
(6 May, 2024) GIBF announced today that it has invested $10 million in Nectin Therapeutics Ltd, proceeds from financing to support ongoing clinical development of lead program targeting PVR as well as IND-enabling activities for multiple ADC candidates.

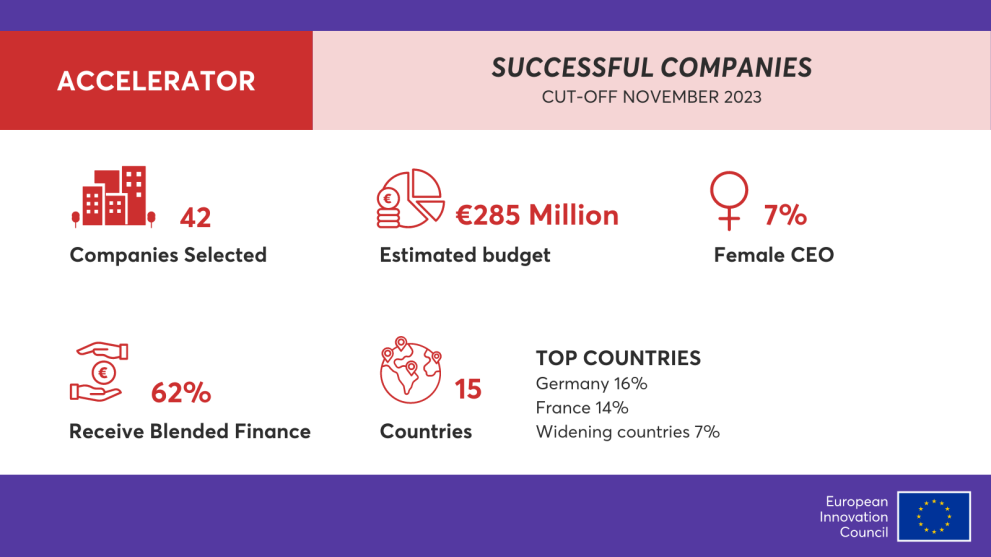
Breaking news - Nectin winning the EIC funding
(18 March, 2024) Nectin Therapeutics Ltd. (" Nectin "), the parent company of Guangzhou Nectin Pharma Ltd. has been selected as one of the 42 recipients of EIC funding in the 2023 EIC Accelerator from a competitive field of 1,083 proposals and 242 companies that were reviewed.

New partnership between 20/20NOW and Visionix Americas
(16 Nov, 2023) Visionix USA is proud to share they recently entered a strategic alliance with 20/20NOW to help bring cost effective, in-office, quality synchronous tele-optometry comprehensive eye exams and ocular telemedicine closer to more patients throughout the United States.


Silexion Therapeutics Presented The LODER™ Phase 2 Summary Results In The 2023 ESMO Immuno-Oncology Congress
(30 Oct, 2023) Results from a multinational Phase 2 clinical study (Protact Trial) of siRNA directed against the oncogenic KRAS G12D and G12V mutations in an extended release formulation (Loder™) was presented at the 2023 European Society for Medical Oncology (ESMO) annual congress (Abstract 1626P).


FDA Clears CorNeat EverPatch, World's First Non-Degradable Synthetic Tissue Substitute for Ophthalmic Surgery
(8 Jun, 2023) CorNeat Vision's EverPatch utilizes EverMatrix™, a novel material technology able to integrate with surrounding tissue


Kamari Pharma Raises $8 Million from the GIBF2 Fund
(18 Sep, 2022) Kamari Pharma announced the completion of an $8 million investment by the Chinese fund GIBF2.


GIBF Completed a $ 4 Million Investment in CorNeat Vision
(11 Sep, 2021) GIBF recently completed a $ 4 million investment in a subsidiary of CorNeat Vision, which was established in Guangzhou, China.


GIBF Raised 300 million Dollars on the Second Fund
(31 May 2021) The Guangzhou Bio-industry Investment Fund, has already closed $200 million for its second fund to finance clinical trials.

